Multiply your rewards and
Help us continue to provide FREE,
quality healthcare
for everyone in Pakistan.
We treat over 400,000
patients monthly for FREE.
70% of our patients are
Zakat Eligible.
We’re making healthcare accessible to millions of vulnerable and underserved children, men and women in Pakistan, and doing it absolutely free-of-cost.
But we can’t do it alone. Become a monthly donor today and help save lives. Your donation will be used for the highest priority needs of Indus Hospital & Health Foundation
But we can’t do it alone.
Become a monthly donor today and help save lives.
#HealthFirst

400,000+
Patients Benefit Per Month.
10,500+
Children treated for
cancer & blood diseases.
Zero Cost
To patients & their family.

400,000+
Patients Benefit Per Month.

10,500+
Children treated for
cancer & blood diseases.

Zero Cost
To patients & their family.
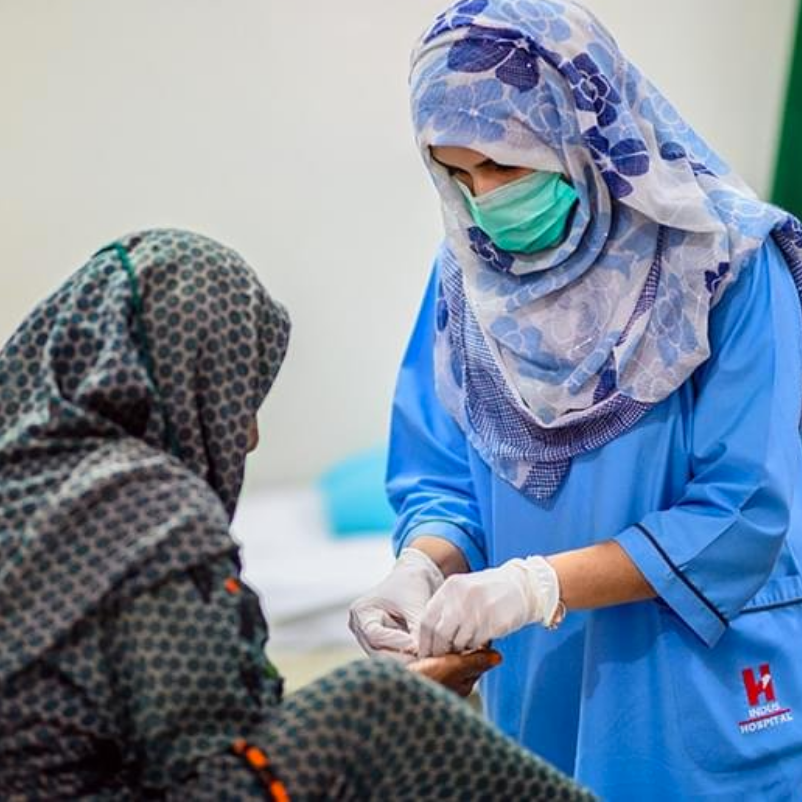
Primary Care
100 million Pakistanis havenever visited the doctor
Imagine being sick and not being able to visit the doctor.
Imagine not being able to have regular checkups. Imagine living a life with an illness - but never knowing which one, or how to cure it.
This is the sad reality for millions of Pakistanis - and why supporting our Primary Care Program (PCP) is so important.
We can provide it for FREE, but we need YOUR help.
Primary Care
100 million Pakistanis have never visited the doctor
Imagine being sick and not being able to visit the doctor. Imagine not being able to have regular checkups. Imagine living a life with an illness - but never knowing which one, or how to cure it.
This is the sad reality for millions of Pakistanis - and why supporting our Primary Care Program (PCP) is so important.
We can provide it for FREE, but we need YOUR help.
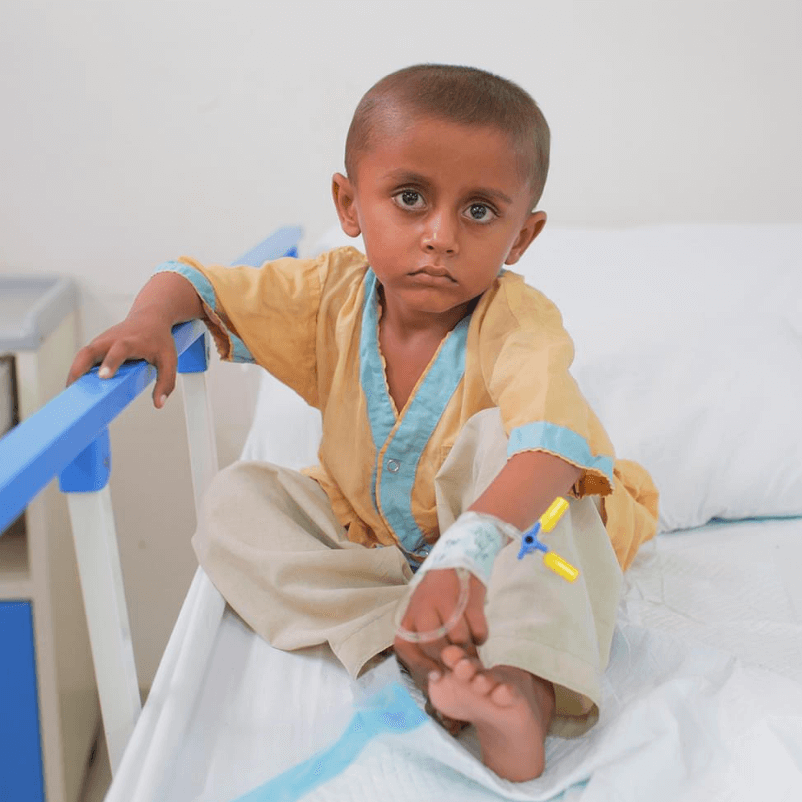
Childhood Cancer
Around 8,000 children in Pakistan under the age of 18 are diagnosed with cancer every year. Due to expensive treatment, most families cannot afford it.
Imagine being a parent and feeling helpless because you can’t afford treatment for your sick child. Every child deserves a chance to live life to the fullest, to grow up, and experience the joys of childhood.
Fortunately, more than 80% of times, childhood cancer is permanently curable if proper treatment is given at an early stage.
Just like treatment to other diseases, Indus Hospital has been leading the way in bringing hope for children suffering with cancer.
One chemotherapy session costs only £25.
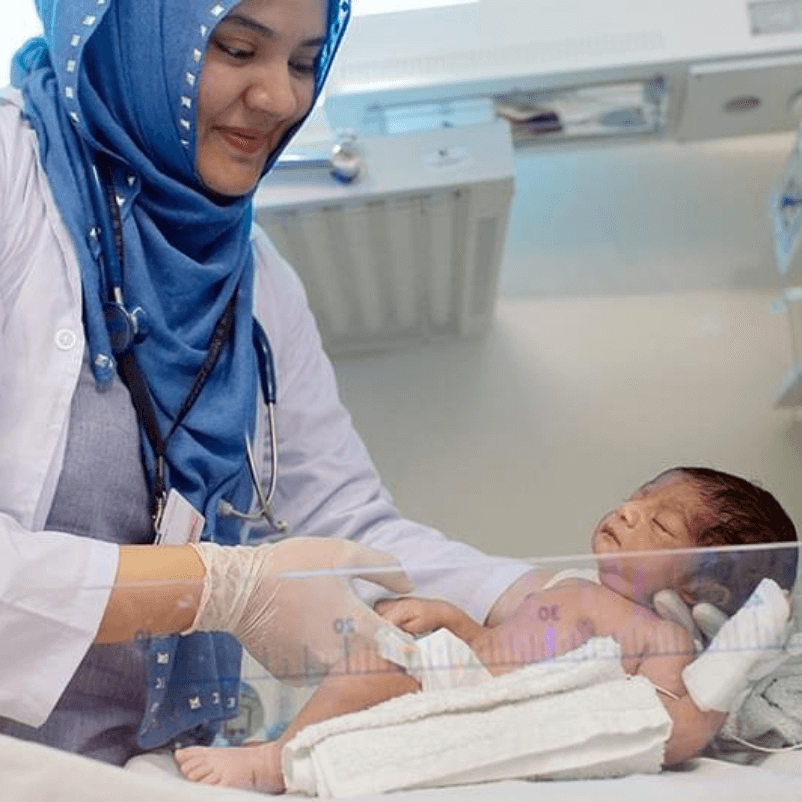
Mother & Child
Pakistan has one of the highest
maternal mortality ratios in South Asia.
For many, experiencing pregnancy, childbirth and motherhood is a blessing. Unfortunately, there are thousands of women in Pakistan who struggle through this important chapter of their lives.
- No prenatal care
-
Lack of adequate options for safe delivery
-
High risk of maternal mortality
-
Other risks associated with births
-
Lack of postpartum care.
A wonderful experience only to be taken away because of a lack of access to healthcare. We can provide it for FREE and save lives, but we need YOUR help.
Pakistan has one of the highest
maternal mortality ratios in South Asia.
For many, experiencing pregnancy, childbirth and motherhood is a blessing. Unfortunately, there are thousands of women in Pakistan who struggle through this important chapter of their lives.
- No prenatal care
-
Lack of adequate options for safe delivery
-
High risk of maternal mortality
-
Other risks associated with births
-
Lack of postpartum care.
A wonderful experience only to be taken away because of a lack of access to healthcare. We can provide it for FREE and save lives, but we need YOUR help.
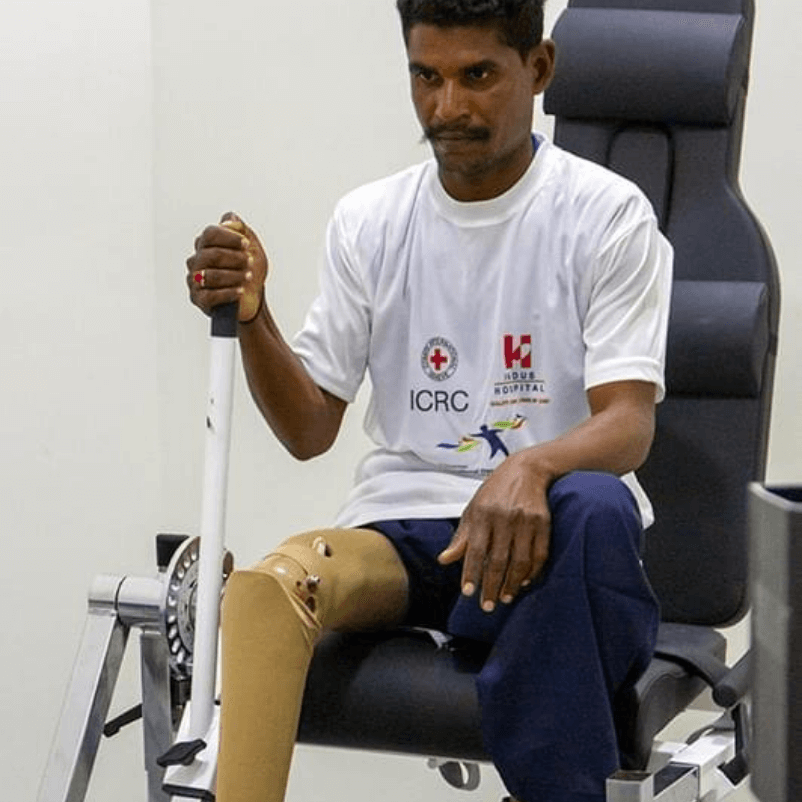
Physical Rehabilitation
Every single person deserves the chance to live life to the fullest.
Unfortunately, those with physical disabilities in Pakistan are often disregarded or brushed aside. They’re not given the same opportunities that able-bodied individuals are given. We can give them a second chance, but we need YOUR help.
Indus Hospital & Health Network is the only hospital in Pakistan providing FREE-of-cost prosthetic limbs along with physiotherapy to help patients walk again. Additionally, the facility also provides psychological therapy to patients and their families to help them cope with the circumstances.
And so much more
And so much more
Besides the above programs and initiatives, IHHN also operates: Blood clinics, Infectious disease awareness and treatment programs, Education, training and research facilities.
Open your heart, be generous with your donations and zakat
SECURE GIVING | ZAKAT ELIGIBLE
CUSTOM JAVASCRIPT / HTML
CUSTOM JAVASCRIPT / HTML
CUSTOM JAVASCRIPT / HTML

Your Zakat is their Lifeline.
“And establish prayer and give Zakat, and whatever good
you put forward you put forward for yourselves
- you will find it with Allah.” (2:110)
More than 70% of patients benefiting from our services are Zakat eligible.
Open your heart. Be generous with your Zakat.
Your Zakat is their Lifeline.
“And establish prayer and give Zakat, and whatever good you put forward you put forward for yourselves - you will find it with Allah.” (2:110)
More than 70% of patients benefiting from our services are Zakat eligible. Open your heart. Be generous with your Zakat.
How Zakat is utilized at Indus Hospital & Health Network:
- Based on the policy and guidelines provided by IHHN’s Shariah Supervisory Committee and Zakat Committee, Zakat fund is solely used for the treatment of eligible patients – the Mustehqin.
- Zakat funds are only used for eligible zakat recipients.
- The disbursement of the fund is through a transparent process where the patient allows the Hospital to use Zakat for his/her treatment.
-
To ensure our donors have transparency over the utilization of their Zakat, audited accounts are publicly available.
Zakat Calculator:
CUSTOM JAVASCRIPT / HTML

Some of our supporters
Shaykh Omar Suleiman
Ustadha Yasmin Mogahed
Imam Siraj Wahhaj
Shaykh Alaa Elsayed
Shaykh Musleh Khan
Fawad Khan
Bushra Ansari
Sanam Jung
Momal Sheikh & Imran Abbas
The Story of Indus Hospital by Dr. Abdul Bari Khan
Our mission: free-of-cost, quality healthcare for all.
Our mission: FREE-of-cost, quality healthcare for all.
About Indus Health Network
Is Indus Health Network UK and The Indus Hospital, Pakistan the same?
No, they are both different entities. The Indus Hospital is a hospital based in Korangi, Karachi which provides quality medical services FREE of cost to all irrespective of religion, caste, creed, age, gender and financial status.
Indus Health Network UK is a registered charity with UK Charity Commission – 1154809. The purpose of the IHN UK is to create awareness amongst the expat Pakistanis, professionals and donor agencies to support the Indus Hospital & Health Network and its cause to provide FREE of cost quality healthcare to the underserved community of Pakistan.
Does Indus Hospital charge any money from the patients?
No, the patients at the Indus Hospital are charged £0 for every visit and treatment.
What are the ways I can donate to Indus Hospital?
You can donate to the Indus Hospital in multiple ways including:
- PayPal
-
Credit Card
-
Donate to our campaign
-
Feeling Blessed
-
Double your Donation/Matching Gift
-
Amazon Smile
-
Call us to Donate
Why support Indus Hospital & Health Network
What is childhood cancer?
Childhood cancer (also called pediatric cancer) typically refers to a cancer that is found in children and teens, and sometimes young adults. It is not just one disease. There are many types, which can be found in different places throughout the body.
The most common cancer in children is leukemia, a type of blood cancer. Cancer can also occur in organs and tissues such as the lymph nodes (lymphoma), nervous system (brain tumors) and muscles, bone and skin (solid tumors).
Does cancer treatment save lives?
Cancer treatments do in fact regularly save lives. However, one of the challenges of a cancer diagnosis is the need for continued treatment making your support all the more necessary for those who have nowhere to turn.
Is my donation Zakat eligible?
Yes! Approximately 70% of our recipients are Zakat Eligible.
What is the average cost of cancer treatment?
You can cover the entire treatment for a child for £3000 GBP!
How is my Zakat money spent? Who is it spent on?
What are your guidelines for distributing Zakat Donations?
The Indus Hospital provides quality care without any discrimination, FREE of cost through the support of your Zakat and Donations.
With the help of reputable Sharia Scholars, The Indus Hospital (TIH) has developed a criterion to ascertain if Zakat is applicable to a certain individual. Whenever a patient visits the Indus Hospital, he/she goes through the assessment with TIH’s welfare officer to determine if the patient is eligible for Zakat or not.
Once the recipient of Zakat is identified, their MR number is marked to signify that the patient’s treatment should be supported from Zakat account. The patient is requested to sign a declaration granting the Indus Hospital permission to collect Zakat on their behalf and to use that Zakat to sponsor the treatment of his/her and/or other patients or hospital requirements. Once the patient is discharged, hospital generates an internal bill and sends it to the Zakat Committee for settlement.
Indus Health Network UK is a registered charity with UK Charity Commission – 1154809. The purpose of the IHN UK is to create awareness amongst the expat Pakistanis, professionals and donor agencies to support the Indus Hospital & Health Network and its cause to provide free of cost quality healthcare to the underserved community of Pakistan.